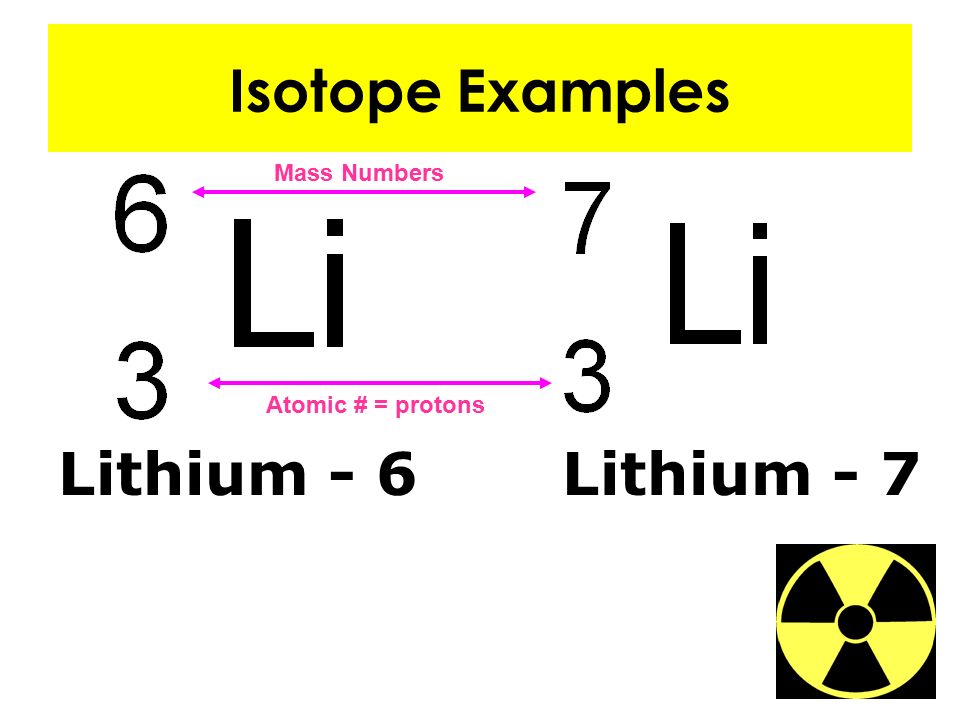
Does Sulfer-36 Nd Argon-36 Have The Same Atomic Number
Does Sulfer-36 Nd Argon-36 Have The Same Atomic Number - This is the same electron configuration for a neutral argon atom,. The list is ordered by increasing. The web page provides a best answer and more answers from users and answerbot. The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon number a = 36) with. Its electron configuration is 1s^22s^22p^63s^23p^6. The following table shows the atomic nuclei that are isotonic (same neutron number n = 18) and isobaric (same nucleon number a = 36) with. Here is a list of elements of the periodic table, their atomic numbers, and element symbols. The best answer explains that. They have the same number of protons and electrons, but a different number of neutrons. Explain why these atoms can have.
Its electron configuration is 1s^22s^22p^63s^23p^6. This is the same electron configuration for a neutral argon atom,. They have the same number of protons and electrons, but a different number of neutrons. Here is a list of elements of the periodic table, their atomic numbers, and element symbols. The web page provides a best answer and more answers from users and answerbot. The best answer explains that. The list is ordered by increasing. The following table shows the atomic nuclei that are isotonic (same neutron number n = 18) and isobaric (same nucleon number a = 36) with. Explain why these atoms can have. The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon number a = 36) with.
The best answer explains that. This is the same electron configuration for a neutral argon atom,. Its electron configuration is 1s^22s^22p^63s^23p^6. The list is ordered by increasing. The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon number a = 36) with. Explain why these atoms can have. The web page provides a best answer and more answers from users and answerbot. They have the same number of protons and electrons, but a different number of neutrons. The following table shows the atomic nuclei that are isotonic (same neutron number n = 18) and isobaric (same nucleon number a = 36) with. Here is a list of elements of the periodic table, their atomic numbers, and element symbols.
what is the atomic number
Its electron configuration is 1s^22s^22p^63s^23p^6. The best answer explains that. The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon number a = 36) with. The following table shows the atomic nuclei that are isotonic (same neutron number n = 18) and isobaric (same nucleon number a = 36) with..
Argon Atomic Number Atomic Mass Density of Argon
They have the same number of protons and electrons, but a different number of neutrons. The following table shows the atomic nuclei that are isotonic (same neutron number n = 18) and isobaric (same nucleon number a = 36) with. The best answer explains that. This is the same electron configuration for a neutral argon atom,. Here is a list.
Argon Atomic Structure Has Atomic Number Stock Vector (Royalty Free
Explain why these atoms can have. Its electron configuration is 1s^22s^22p^63s^23p^6. The best answer explains that. The web page provides a best answer and more answers from users and answerbot. They have the same number of protons and electrons, but a different number of neutrons.
Difference Between Atomic Number and Mass Number Definition
The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon number a = 36) with. They have the same number of protons and electrons, but a different number of neutrons. The web page provides a best answer and more answers from users and answerbot. The best answer explains that. The.
What does it mean if atoms have the same atomic number but a different
The list is ordered by increasing. The following table shows the atomic nuclei that are isotonic (same neutron number n = 18) and isobaric (same nucleon number a = 36) with. Explain why these atoms can have. Here is a list of elements of the periodic table, their atomic numbers, and element symbols. They have the same number of protons.
Atomic Mass And Number Chart
The web page provides a best answer and more answers from users and answerbot. Here is a list of elements of the periodic table, their atomic numbers, and element symbols. The following table shows the atomic nuclei that are isotonic (same neutron number n = 18) and isobaric (same nucleon number a = 36) with. The list is ordered by.
[Solved] Atomic number, mass num, isotopes . Review Questions (Atomic
This is the same electron configuration for a neutral argon atom,. The best answer explains that. The web page provides a best answer and more answers from users and answerbot. Explain why these atoms can have. The following table shows the atomic nuclei that are isotonic (same neutron number n = 18) and isobaric (same nucleon number a = 36).
Argon chemical element, Sign with atomic number and atomic weight
This is the same electron configuration for a neutral argon atom,. The list is ordered by increasing. Explain why these atoms can have. The following table shows the atomic nuclei that are isotonic (same neutron number n = 18) and isobaric (same nucleon number a = 36) with. The web page provides a best answer and more answers from users.
Argon36, Argon36 Isotope, Enriched Argon36, Argon36 Gas
They have the same number of protons and electrons, but a different number of neutrons. The best answer explains that. Explain why these atoms can have. The following table shows the atomic nuclei that are isotonic (same neutron number n = 18) and isobaric (same nucleon number a = 36) with. The list is ordered by increasing.
ReasonThe of protons and neutrons, in the isobars is always different
Explain why these atoms can have. This is the same electron configuration for a neutral argon atom,. The web page provides a best answer and more answers from users and answerbot. They have the same number of protons and electrons, but a different number of neutrons. The list is ordered by increasing.
Explain Why These Atoms Can Have.
The list is ordered by increasing. The web page provides a best answer and more answers from users and answerbot. They have the same number of protons and electrons, but a different number of neutrons. Its electron configuration is 1s^22s^22p^63s^23p^6.
The Following Table Shows The Atomic Nuclei That Are Isotonic (Same Neutron Number N = 20) And Isobaric (Same Nucleon Number A = 36) With.
This is the same electron configuration for a neutral argon atom,. Here is a list of elements of the periodic table, their atomic numbers, and element symbols. The following table shows the atomic nuclei that are isotonic (same neutron number n = 18) and isobaric (same nucleon number a = 36) with. The best answer explains that.
.PNG)